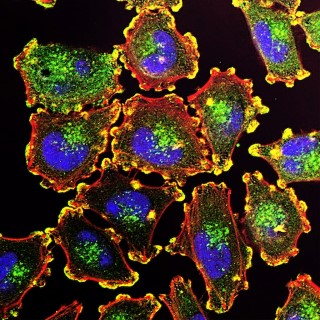
Induced pluripotent stem cells (otherwise known as iPSCs) are derived from adult somatic cells and reprogrammed by inducing factors and genes to be pluripotent. They’re akin to embryonic stem cells in several ways, and with the recent progresses in iPSC-based therapy and reprogramming, scientists have noted huge potential for these cells in disease modeling and personalized medicine.
Alice Chang, Ph.D. explains how, discovered in 2006 by Yamanaka lab at Kyoto University in late 2007, this healthcare breakthrough allowed researchers to garner pluripotent stem cells without the ethically gray usage of embryos, offering a powerful method to de-differentiate cells. Incredibly, tissues derived from these cells are a near-identical match to the cell donor.
Creating iPS Cells
These days, professionals use many different methods to reprogram somatic cells into iPSCs.
Initially, genomic integration coupled with high expression of four factors could form iPS cells from fibroblast cells. However, continued research shows they can actually be generated from fibroblasts by integration of just three of the four factors (namely Oct4, Klf4, and Sox2). Such studies have noted that ectopic expression of the three factors is necessary for transforming somatic cells into induced pluripotent stem cells.
In more recent history, scientists have discovered different growth factors and compounds that can improve reprogramming efficiency, like combining BIX-01294 and BayK8644s with Oct4/Klf4 to derive iPSCs from mouse embryonic fibroblasts.
Furthermore, retroviruses have been utilized to reprogram somatic cells, showcasing their effectiveness for amalgamating exogenous genes into somatic cells’ genomes to create both mouse and human iPSCs. Although, retroviral vectors may come with too-significant risks to end up being wholly useful for patients.
Luckily, several strategies were created in response to address this issue. One of the most popular is the implementation of CRISPR-Cas9 technology, enabling iPSCs generation with next to no genetic mutation.
Induced Pluripotent Stem Cells Potential in Disease Modeling
Induced pluripotent stem cells are invaluable when it comes to disease modeling, allowing professionals to create patient-specific models to accelerate research in precision medicine. The following are the most prevalent:
- Parkinson’s disease iPSC model — These models avoid the likelihood of species differences between mice and humans. Plus, they’re derived from PD patients, so iPSCs capture the complete picture of genetic risk factors, rather than the simplicity of the cellular and animal models previously used.
- Cardiomyopathy iPSC model — With models derived from iPSCs, scientists can recapitulate the pathological earmarks of hypertrophic cardiomyopathy instead of relying on rodent models that don’t provide a comprehensive overview.
iPSCs Show Promise for Generating Patient-Specific Cells in Personalized Treatments
Otherwise dubbed precision medicine, personalized medicine provides tailored treatment that considers the genetic, clinical, and environmental characteristics of each patient, providing quite substantial benefits over conventional medicine.
The application of iPSCs in this field shows potential for personalized cancer therapy, drug development, and tissue regeneration. Various reprogramming and expansion techniques are being studied to make the utilization off iPSCs in personalized medicine into a widespread phenomenon. While it will certainly take a long time for this to take effect, it’s no less exciting to those working in the field.